A. Lengkapilah tabel berikut dengan rumus kimia beserta nama senyawa yang benar
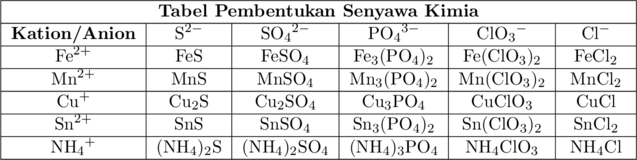
\(\ce{FeS}\) : besi (II) sulfida atau ferro sulfida
\(\ce{FeSO4}\) : besi (II) sulfat atau ferro sulfat
\(\ce{Fe3(PO4)2}\) : besi (II) fosfat atau ferro fosfat
\(\ce{Fe(ClO3)2}\) : besi (II) klorat atau ferro klorat
\(\ce{Fe(Cl)2}\) : besi (II) klorida atau ferro klorida
\(\ce{MnS}\) : mangan (II) sulfida atau mangano sulfida
\(\ce{MnSO4}\) : mangan (II) sulfat atau mangano sulfat
\(\ce{Mn3(PO4)2}\) : mangan (II) fosfat atau mangano fosfat
\(\ce{Mn(ClO3)2}\) : mangan (II) klorat atau mangano klorat
\(\ce{Mn(Cl)2}\) : mangan (II) klorida atau mangano klorida
\(\ce{Cu2S}\) : tembaga (I) sulfida atau cupro sulfida
\(\ce{Cu2SO4}\) : tembaga (I) sulfat atau cupro sulfat
\(\ce{Cu3PO4}\) : tembaga (I) fosfat atau cupro fosfat
\(\ce{CuClO3}\) : tembaga (I) klorat atau cupro klorat
\(\ce{CuCl}\) : tembaga (I) klorida atau cupro klorida
\(\ce{SnS}\) : timah (II) sulfida atau stano sulfida
\(\ce{SnSO4}\) : timah (II) sulfat atau stano sulfat
\(\ce{Sn3(PO4)2}\) : timah (II) fosfat atau stano fosfat
\(\ce{Sn(ClO3)2}\) : timah (II) klorat atau stano klorat
\(\ce{SnCl2}\) : timah (II) klorida atau stano klorida
\(\ce{(NH4)2S}\) : amonium sulfida
\(\ce{(NH4)2SO4}\) : amonium sulfat
\(\ce{(NH4)3PO4}\) : amonium fosfat
\(\ce{NH4ClO3}\) : amonium klorat
\(\ce{NH4Cl}\) : amonium klorida
B. Tuliskan nama setiap senyawa berikut
1. \(\ce{Au2S3}\)
2. \(\ce{SO3}\)
3. \(\ce{CH3COOH}\)
4. \(\ce{CaI2}\)
5. \(\ce{K2Cr2O7}\)
6. \(\ce{MgH2}\)
7. \(\ce{PCl5}\)
8. \(\ce{KMnO4}\)
9. \(\ce{N2O5}\)
10. \(\ce{HClO}\)
11. \(\ce{SnO2}\)
12. \(\ce{H3AsO4}\)
13. \(\ce{K2SiO3}\)
14. \(\ce{CaCO3}\)
15. \(\ce{H2S2O3}\)
16. \(\ce{CH4}\)
17. \(\ce{C6H12O6}\)
18. \(\ce{NH4Br}\)
19. \(\ce{CH3COOK}\)
20. \(\ce{C2H5OH}\)
21. \(\ce{NaHSO4}\)
22. \(\ce{MgC2O4}\)
23. \(\ce{Hg2SO4}\)
24. \(\ce{MgO2}\)
25. \(\ce{H3BO3}\)
- Emas (III) sulfida
- Belerang trioksida
- Asam asetat
- Kalsium iodida
- Kalium dikromat
- Magnesium hidrida
- Fosfor pentaklorida
- Kalium permanganat
- Dinitrogen pentaoksida
- Asam hipoklorit
- Timah (IV) oksida
- Asam arsenat
- Kalium silikat
- Kalsium karbonat
- Asam tiosulfat
- Metana
- Glukosa
- Amonium bromida
- Kalium asetat
- Etanol
- Natrium bisulfat
- Magnesium oksalat
- Raksa (I) sulfat
- Magnesium peroksida
- Asam borat
C. Tuliskan rumus senyawa berikut
- Amonium sulfat
- Asam formiat
- Stano klorida
- Mangano sulfat
- Ferri klorida
- Asam perklorat
- Natrium tiosulfat
- Potassium karbonat
- Aluminium antimonida
- Sodium dikromat
- Timah (IV) sulfida
- Magnesium asetat
- Kalium silikat
- Aluminium hidroksida
- Amonium hidroksida
- Sukrosa
- Urea
- Boron trifluorida
- Ozon
- Plumbi hidroksida
1. \(\ce{(NH4)2SO4}\)
2. \(\ce{HCOOH}\)
3. \(\ce{SnCl2}\)
4. \(\ce{MnSO4}\)
5. \(\ce{FeCl3}\)
6. \(\ce{HClO4}\)
7. \(\ce{Na2S2O3}\)
8. \(\ce{K2CO3}\)
9. \(\ce{AlSb}\)
10. \(\ce{Na2Cr2O7}\)
11. \(\ce{SnS2}\)
12. \(\ce{Mg(CH3COO)2}\)
13. \(\ce{K2SiO3}\)
14. \(\ce{Al(OH)3}\)
15. \(\ce{NH4OH}\)
16. \(\ce{C12H22O11}\)
17. \(\ce{CO(NH2)2}\)
18. \(\ce{BF3}\)
19. \(\ce{O3}\)
20. \(\ce{Pb(OH)4}\)
